One of the biggest challenges pharmaceutical companies face is keeping up with changing regulations by state and federal organizations. This can be confusing as some agency rulings are established with specific milestones that can span several years, and the requirements for meeting each milestone may also be complex.
The Drug Supply Chain Security Act (DSCSA) is a prime example. Enacted by Congress on November 27, 2013, the regulation includes milestones that began in 2014 and continue through 2023. In 2018, many companies scrambled to comply with the deadline of providing unique product or serial codes on all prescription products. Many requirements will continue to be rolled out in phases with the deadline of full traceability via an electronic system by 2023. All parties in the supply chain should work together, meeting each milestone along the way to reach this goal.
The purpose of the DSCSA is to provide a “track and trace” system that allows every drug in the U.S. to be traced from manufacturing through to the end user. The primary goal of the regulation is to ensure that all prescription drugs that reach the patient are safe and effective, and to make it easier to identify illegal or counterfeit drugs as they enter the supply chain. When the act is fully in force in 2023, every pharmaceutical product will bear a unique identifying code or number allowing it to be traced all the way through the supply chain.
How Can Pharmaceutical Companies Remain Compliant?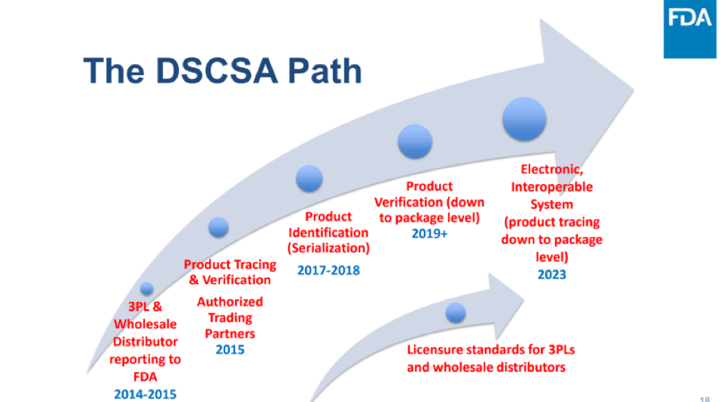
Every entity that deals with a drug is covered by the DSCSA. Manufacturers will be tasked with ensuring that the appropriate tracking methods are used on each product, transportation companies will need to document each step of the supply chain, and pharmacists must be able to track the entire path of the drug prior to dispensation. There are three main ways that companies can stay compliant:
Documentation
The most important document is the T3–the DSCSA Transaction Report. The report is broken into three main sections: transaction information, transaction history, and transaction statement. The T3 must always be available. Whether an inspection is random or motivated by suspicion, inspectors may choose any product and ask to see the relevant T3.

Verification
Some inspections may take place because a product in the pharmacist’s possession may be suspect. If this happens, the pharmacist must be prepared to produce supporting evidence for every step and every date set out in the T3.
Penalties
A pharmacist unable to produce the required T3 documentation, or who produces inaccurate or inadequate documentation, may face penalties. Pharmacists may be fined and product may also be quarantined. Transporters, distributors, and manufacturers may also be penalized depending on the severity of the violation.
Supply Chain Responsibility
As well as implementing a recording, archival, and recovery system allowing T3s to be produced at any point, pharmacists must also closely examine all T3s to ensure accurate transaction reporting and that nothing in the T3 would give an observant, informed, and cautious recipient any reason to question the authenticity of the chain of transactions or of the legitimacy of the drug itself. The DSCSA is a comprehensive set of regulations that will impact every stage of a pharmaceutical product’s lifecycle. Remaining compliant will require companies to have a systemic approach to meeting each of the core rules.
