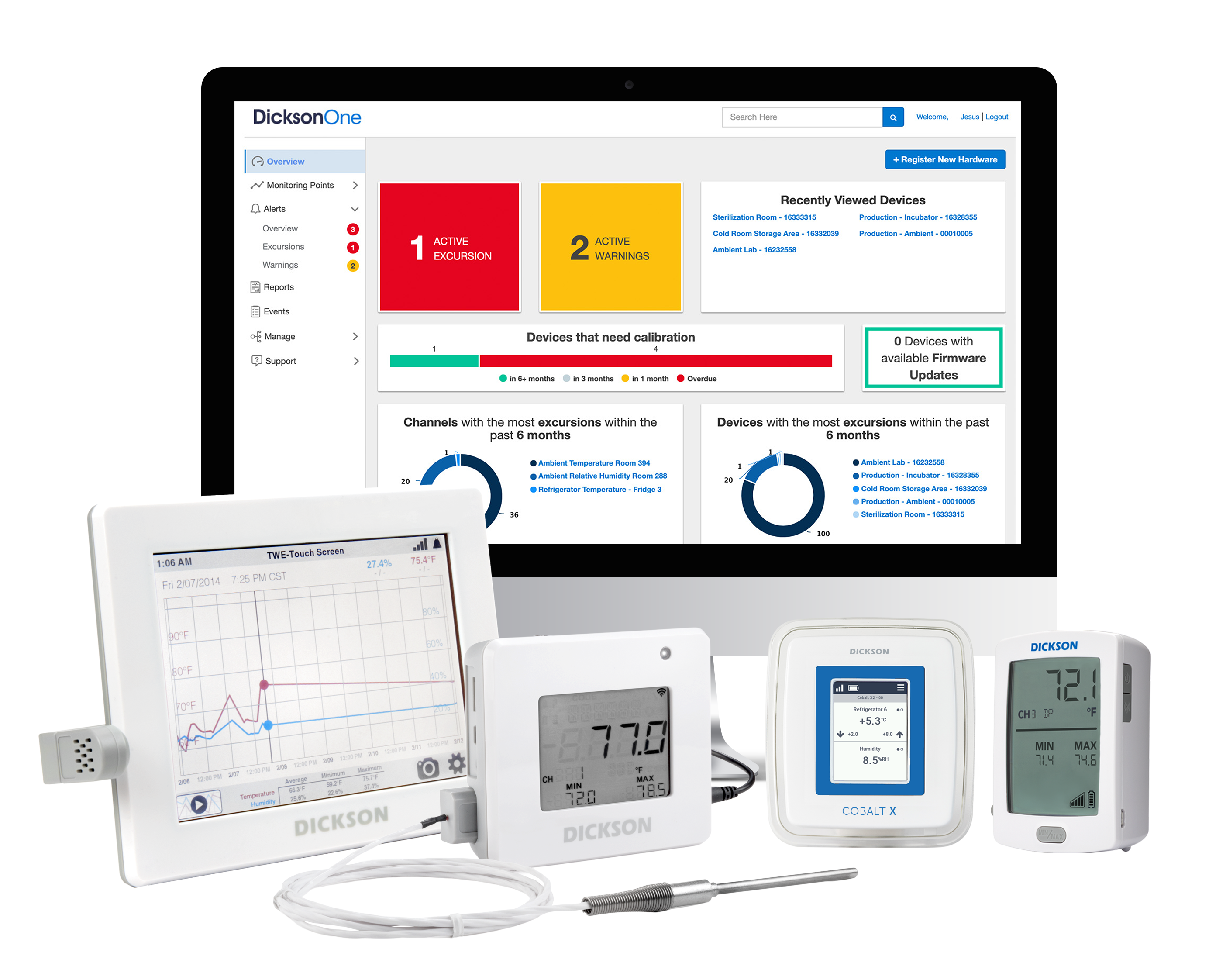
In 2019, Oceasoft and Dickson merged and began to combine their expertise and resources. The two companies have formed a powerful alliance offering unparalleled capabilities in data collection, analysis, and reporting. Dickson Data's extensive experience in temperature and humidity monitoring and...
Read MorePoor storage conditions that expose delicate pharmaceuticals and biologics to changes in temperature and humidity can be very costly in the form of lost product, poor end quality for patients, and damage to reputation.
What Defines Pharmaceutical Product Quality?
Pharmaceutical products must be...
Read MoreFrom a decade ago, cloud computing has come a long way – spreading to a wide array of industries. Recently, the highest amount of IT investment in life sciences industry has been on cloud computing. The cost and time commitment to develop a new drug nearly doubles every nine years. Pharma and...
Read MoreWhat is Cold Chain Management?
Many products in the pharmaceutical industry, such as modern biologics, monoclonal antibodies, vaccines, gene therapies, some types of insulin, and some cancer treatments require strict adherence to a precise temperature range at all times. This cold chain...
Read MoreA growing trend in the pharmaceutical industry is the development of more complex and fragile products, like biologicals, chemical mixtures, and large molecules (COVID-19 vaccines are an example of this trend).
Read MoreWhy is Pharmacy Temperature Monitoring so Important?
If medications and vaccines are stored at the wrong temperature, several things can go wrong – rendering them less effective than intended or even chemically altered, causing inadvertent patient harm. Because of this risk, pharmacy regulations...
Read MoreSince their introduction, IoT devices have evolved from technical curiosities to ubiquitous elements in daily life, finding applications in home automation, healthcare, manufacturing, agriculture, traffic control, and a diverse collection of other areas.
Read MoreA central aspect of doing business in highly regulated industries, like pharmaceutical manufacturing and distribution, healthcare, or aerospace, is the need to have extensive systems in place to protect consumers and maintain regulatory compliance. That includes systems to monitor the environmental...
Read More