Companies operating in highly regulated industries are no strangers to FDA regulations, audits, inspections and noncompliance. Regulations cover a wide range of topics, from product labeling to environmental conditions to manufacturing practices. Companies operating in the medical device,...
Read Moreinsights
The United States Pharmacopeial Convention, or USP, is a nonprofit science-driven organization that establishes standards for medicines. These standards are developed with independent experts and are primarily published in a compendium of General Chapters. While USP deals with quality and safety,...
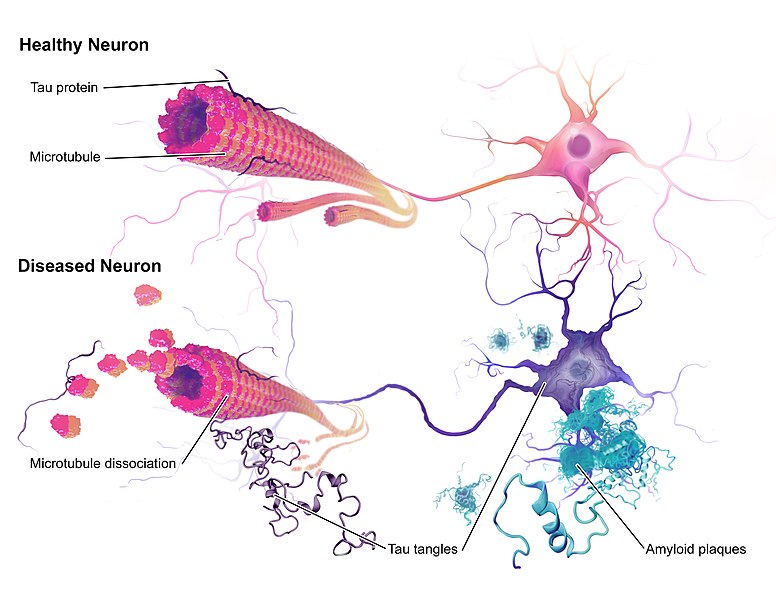
Read MoreMany people have a connection to Alzheimer’s in some way. After all, the memory disorder affects over 43 million people worldwide. For anyone who has witnessed the disease firsthand, the impact on quality of life can be devastating. It’s no wonder, then, that so many resources – funding and...
Read MoreIn March 2019, the U.S. House Aviation Subcommittee held a hearing discussing the future of aviation. The hearing, entitled “Looking Forward: Aviation 2050”, met to discuss the potential evolution of the industry and explore how emerging technologies will change the domestic airspace.
Read MoreIn healthcare, surgery is used to diagnose and treat a range of ailments. Every operation requires anesthesia, and every time anesthesia is used, the exhaled fumes are removed from the operating room and released into the atmosphere. Most surgeries use either sevoflurane or desflurane, and...
Read More